One way to obliterate an atom is to shoot it with the planets most powerful X-ray gun. Linda Young tried that experiment in October 2009, when she was testing the newly opened X-ray free-electron laser at the SLAC National Accelerator Laboratory in Menlo Park, California. A single pulse from the US$420-million machine packs the same energy as all the solar radiation hitting Earth at that moment, but focused down to one square centimetre. “It will destroy anything you put in front of it,” says Young.
When the laser pulse slammed into the neon atoms in that experiment, it made them explode, stripping away each atoms 10 electrons within 100 femtoseconds (1 femtosecond is 10−15 seconds). But it was the manner of this destruction that most interested Young, who heads the X-ray science division at Argonne National Laboratory in Illinois. The X-rays first removed the atoms inner electrons, leaving the outer ones in place. For a brief moment, the neon atoms in the path of the laser became hollow.
Free podcast
Richard Van Noorden discusses some exotic forms of atom
That exotic form of neon is one of a number of strange species created by physicists intent on contorting atoms. Some teams have inflated atoms to the size of dust particles. Several research collaborations are creating anti-atoms out of antimatter. And others have loaded atomic nuclei with protons and neutrons in the quest to forge new superheavy elements. Some of the experiments aim to investigate atomic structure; others use atoms as the first steps in modelling more complicated systems. They are all descendants of the revolution in atomic theory catalysed by Danish physicist Niels Bohr 100 years ago. But Bohr would have had difficulty imagining how far scientists could go in poking and prodding atoms into such extreme forms.
Hollow atoms

THOMAS POROSTOCKY
The atom that Bohr proposed1 in July 1913 looked like a miniature Solar System, with electrons arranged in concentric orbits around a positively charged nucleus. In Bohrs model, electrons were point-like particles that were quantized, meaning that they could jump from one orbit to another but could not exist in between. The advent of quantum mechanics in the 1920s retained the concept of orbits but re-imagined electrons as spreading everywhere around the nucleus. The location of each electron can be described only in probabilities, in the form of a mathematical wavefunction.
Electrons furthest from the nucleus can be kicked free with the least amount of added energy, so are usually the first to be stripped away. Yet X-rays, which pack a concentrated punch, can remove more tightly bound electrons from inner orbits. A medical X-ray takes out just one of those inner electrons before another from an outer shell drops down to fill the space. But the SLAC X-ray laser is in a class by itself. The beam is so intense and focused that every 100-femtosecond pulse sends 100,000 X-ray photons flying past each square ångström of space (1 ångström is 10−10 metres). That allowed Young to blast away all the inner electrons of the neon atoms in her 2009 experiment2. When electrons from the outer shells dropped into the abandoned inner shells, the beam soon kicked those out as well.
“If you tune your X-rays properly, you can pick which shell you want to empty out first,” says Young. “Being able to control the inner-shell dynamics is very cool.” The current record for this kind of atom-hollowing was reported last November3 by a group at the Center for Free-electron Laser Science in Hamburg, Germany, which used the SLAC laser to strip away, from the inside out, the 36 inner electrons of a 54-electron-strong xenon atom.
Young hopes that research on hollow atoms will prove helpful when the laser is ready for one of its intended uses — creating images of biological molecules such as DNA and proteins by scattering X-rays off their atoms. Those pictures come at a price: the beam quickly destroys the molecules it is imaging. Knowing how hollow atoms form during this process may help researchers to interpret how the scattering pattern changes as a molecule explodes, Young says.
Two decades ago, several research groups made hollow atoms using a different process: first stripping almost all of the electrons from atoms, then depositing the resulting highly charged, slow-moving ions onto a surface. When the ions were a few tens of ångströms away from the surface, they attracted electrons from it, creating momentarily hollow atoms with electrons in outer but not inner shells. Those outer electrons then fell inwards, and the hollow atoms expelled a burst of energetic electrons and photons. “A hollow atom is nothing but a fireball of an enormous amount of energy,” says Joachim Burgdörfer, a physicist at the Vienna University of Technology, who worked on developing the theory of the process4.
Several research groups pursued hollow atoms in the late 1980s and 1990s, with some scientists exploring how the burst of photons from their formation might clean surfaces by removing the topmost layers without doing deeper damage. Although that procedure has been patented, it has not captured the attention of industry, says Fritz Aumayr, a physicist at the Vienna University of Technology. The closest it has come to an application so far was in 2008, when researchers invoked the process to explain how heavy ions spewed from the Sun can damage the surfaces of planets such as Mercury5. The ions become hollow atoms as they drop onto the planet, and release bursts of energy as they land.
This year, Aumayr published a paper6 showing that the energy expelled from ions dropping onto carbon membranes can create nanoscale pores whose size is controlled by the strength of the ions charge (that is, how many electrons it was missing). That might be a useful route for making nanosieves for filtering small molecules, he says, or for creating nanopores to pass DNA through for sequencing.




 دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما هفته پنجم فروردین ماه 1403
دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما هفته پنجم فروردین ماه 1403 دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما هفته اول اردیبهشت ماه 1403
دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما هفته اول اردیبهشت ماه 1403 دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما هفته دوم فروردین ماه 1403
دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما هفته دوم فروردین ماه 1403 دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما فروردین ماه 1403
دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما فروردین ماه 1403 فایل نقشه ی تابلو فرش دستباف طرح طبیعت کلبه زیبا
فایل نقشه ی تابلو فرش دستباف طرح طبیعت کلبه زیبا دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما هفته سوم فروردین ماه 1403
دانلود فایلهای بسته آمادهچاپ و نصب تابلو اعلانات مسجدنما هفته سوم فروردین ماه 1403 برنامه اکسل متره و برآورد،تهیه صورت وضعیت راه و باند سال1403
برنامه اکسل متره و برآورد،تهیه صورت وضعیت راه و باند سال1403 دانلود بسته 8000 تست-عمومی، اختصاصی،تخصصی-استخدامی آموزش پرورش
دانلود بسته 8000 تست-عمومی، اختصاصی،تخصصی-استخدامی آموزش پرورش پاسخنامه کتاب Introductory Steps to Understanding
پاسخنامه کتاب Introductory Steps to Understanding دانلود رایگان کتاب صوتی از سکس تا فراآگاهی
دانلود رایگان کتاب صوتی از سکس تا فراآگاهی دانلود کتاب صوتی روانشناسی تصویر ذهنی ماکسول مالتز
دانلود کتاب صوتی روانشناسی تصویر ذهنی ماکسول مالتز حل مسائل الکترودینامیک کلاسیک جان دیوید جکسون به صورت PDF و به زبان انگلیسی در 214 صفحه
حل مسائل الکترودینامیک کلاسیک جان دیوید جکسون به صورت PDF و به زبان انگلیسی در 214 صفحه![دانلود پاورپوینت در مورد [سلول های بنیادی] - شامل 4 فایل مختلف - قابل ویرایش و ارائه - ppt](https://4kia.ir/s4/img_project/22638_1661011327.jpg) دانلود پاورپوینت در مورد [سلول های بنیادی] - شامل 4 فایل مختلف - قابل ویرایش و ارائه - ppt
دانلود پاورپوینت در مورد [سلول های بنیادی] - شامل 4 فایل مختلف - قابل ویرایش و ارائه - ppt پاورپوینت بازی زندگی است درس 17 تفکر و سواد رسانه ای دهم
پاورپوینت بازی زندگی است درس 17 تفکر و سواد رسانه ای دهم پاورپوینت بز یا سگ تفکر و سبک زندگی هفتم
پاورپوینت بز یا سگ تفکر و سبک زندگی هفتم دانلود جزوه امور مالی و حقوق ثبتی در دفاتر اسناد رسمی
دانلود جزوه امور مالی و حقوق ثبتی در دفاتر اسناد رسمی![دانلود حل المسائل [طراحی و تحلیل آزمایش]: ویرایش هشتم - داگلاس مونتگومری ( 8 ) - زبان انگلیسی - pdf](https://4kia.ir/s4/img_project/22638_1659774456.jpg) دانلود حل المسائل [طراحی و تحلیل آزمایش]: ویرایش هشتم - داگلاس مونتگومری ( 8 ) - زبان انگلیسی - pdf
دانلود حل المسائل [طراحی و تحلیل آزمایش]: ویرایش هشتم - داگلاس مونتگومری ( 8 ) - زبان انگلیسی - pdf دانلود پاورپوینت چیستی انسان 2 درس 10 فلسفه یازدهم انسانی
دانلود پاورپوینت چیستی انسان 2 درس 10 فلسفه یازدهم انسانی دانلود 3 بک دراپ کودک تم فوتبال-کد 8082-8080
دانلود 3 بک دراپ کودک تم فوتبال-کد 8082-8080 پاورپوینت ادبیات بومی 2 درس آزاد فارسی دوازدهم
پاورپوینت ادبیات بومی 2 درس آزاد فارسی دوازدهم پاورپوینت درس 2 علوم تجربی پایه چهارم دبستان (ابتدایی): مخلوط ها در زندگی
پاورپوینت درس 2 علوم تجربی پایه چهارم دبستان (ابتدایی): مخلوط ها در زندگی بسته آموزشی ماساژ کاریز خانواده
بسته آموزشی ماساژ کاریز خانواده مجموعه اسکیس معماری از بناهای ایرانی
مجموعه اسکیس معماری از بناهای ایرانی پاورپوینت درس دهم قرآن هشتم سوره یس، سوره صافات و تفسیر نمونه
پاورپوینت درس دهم قرآن هشتم سوره یس، سوره صافات و تفسیر نمونه سیگنال گرفتن با هوش مصنوعی
سیگنال گرفتن با هوش مصنوعی دانلود رایگان کتاب صوتی روش ها و فنون مشاوره دکتر عبدالله شفیع آبادی
دانلود رایگان کتاب صوتی روش ها و فنون مشاوره دکتر عبدالله شفیع آبادی آموزش نحوه تهیه مدار چاپی
آموزش نحوه تهیه مدار چاپی دانلود پاورپوینت فصل هفتم ریاضی پنجم آمار و احتمال همراه با پاسخ فعالیت ها و تمارین
دانلود پاورپوینت فصل هفتم ریاضی پنجم آمار و احتمال همراه با پاسخ فعالیت ها و تمارین پاورپوینت پدافند غیرعامل درس 10 آمادگی دفاعی نهم
پاورپوینت پدافند غیرعامل درس 10 آمادگی دفاعی نهم دانلود کتاب صوتی روح تسخیر ناپذیر(سفر به ماورای وجود) مایکل سینگر
دانلود کتاب صوتی روح تسخیر ناپذیر(سفر به ماورای وجود) مایکل سینگر فروش فیلتر بورسی استریکلی فقط 75 هزار تومان
فروش فیلتر بورسی استریکلی فقط 75 هزار تومان کسب درآمد اینترنتی 300000 تومان در خانه در کمتر از 30 دقیقه
کسب درآمد اینترنتی 300000 تومان در خانه در کمتر از 30 دقیقه روش درآمدزایی در خواب (تعجب نکنید! کلیک کنید و بخوانید)
روش درآمدزایی در خواب (تعجب نکنید! کلیک کنید و بخوانید) ربات همه کاره اینستاگرام
ربات همه کاره اینستاگرام کسب و کار اینترنتی با درآمد میلیونی
کسب و کار اینترنتی با درآمد میلیونی كسب درآمد اينترنتي روزانه حداقل100هزار تومان تضميني
كسب درآمد اينترنتي روزانه حداقل100هزار تومان تضميني کسب درآمد ابدی و بی نهایت 100% واقعی
کسب درآمد ابدی و بی نهایت 100% واقعی کسب درآمد روزانه حداقل یک میلیون تومان ! کاملا حلال و واقعـی !!
کسب درآمد روزانه حداقل یک میلیون تومان ! کاملا حلال و واقعـی !! مجموعه ی آموزش تعمیر لامپ کم مصرف (از مبتدی تا پیشرفته)
مجموعه ی آموزش تعمیر لامپ کم مصرف (از مبتدی تا پیشرفته) افزایش ممبر کانال، گروه و ربات تلگرام به صورت بی نهایت (اد ممبر)
افزایش ممبر کانال، گروه و ربات تلگرام به صورت بی نهایت (اد ممبر) دانلود پکیج درآمدزایی 400هزارتومن در 40دقیقه (مخصوص شرایط تورم 50 درصدی)
دانلود پکیج درآمدزایی 400هزارتومن در 40دقیقه (مخصوص شرایط تورم 50 درصدی) آموزش بازكردن انواع قفل ها بدون كليد(ويژه)
آموزش بازكردن انواع قفل ها بدون كليد(ويژه) کسب و کار اینترنتی در منزل
کسب و کار اینترنتی در منزل آموزش برنامه نویسی آردوینو
آموزش برنامه نویسی آردوینو دانلود مجموعه آموزشی پایپینگ ( Piping ) و نقشه خوانی + آموزش سه نرم افزار طراحی و تحلیل لوله کشی صنعتی
دانلود مجموعه آموزشی پایپینگ ( Piping ) و نقشه خوانی + آموزش سه نرم افزار طراحی و تحلیل لوله کشی صنعتی بازگردانی پیامک های حذف شده- ریکاوری پیامک ۱۰۰٪ عملی
بازگردانی پیامک های حذف شده- ریکاوری پیامک ۱۰۰٪ عملی آموزش رایگان کسب درآمد از سایت الیمپ ترید ( olymp trade )
آموزش رایگان کسب درآمد از سایت الیمپ ترید ( olymp trade ) اموزش ویرایش امضا و پکیج برنامه اندروید و کسب درامد از مارکت های اندرویدی
اموزش ویرایش امضا و پکیج برنامه اندروید و کسب درامد از مارکت های اندرویدی کد های آماده html و css جهت یادگیری و طراحی سریع
کد های آماده html و css جهت یادگیری و طراحی سریع دانلود نمونه فاکتور آماده با فرمت ورد - اکسل و عکس
دانلود نمونه فاکتور آماده با فرمت ورد - اکسل و عکس آموزش ساخت بازی بدون دانش برنامه نویسی و طراحی سه بعدی مبتدی تا پیشرفته با نرم افزار
آموزش ساخت بازی بدون دانش برنامه نویسی و طراحی سه بعدی مبتدی تا پیشرفته با نرم افزار آموزش كامل تعمير لامپ كم مصرف(اختصاصي)
آموزش كامل تعمير لامپ كم مصرف(اختصاصي) اموزش کسب درامد از اینترنت روزانه ۳میلیون تومان تضمینی و تست شده
اموزش کسب درامد از اینترنت روزانه ۳میلیون تومان تضمینی و تست شده نسخه خطی اشعار و پیشگویی های شاه نعمت الله ولی
نسخه خطی اشعار و پیشگویی های شاه نعمت الله ولی نسخه خطی اشعار و پیشگویی های شاه نعمت الله ولی
نسخه خطی اشعار و پیشگویی های شاه نعمت الله ولی درامدزایی در خواب! (تعجب نکنید! بخوانید)
درامدزایی در خواب! (تعجب نکنید! بخوانید)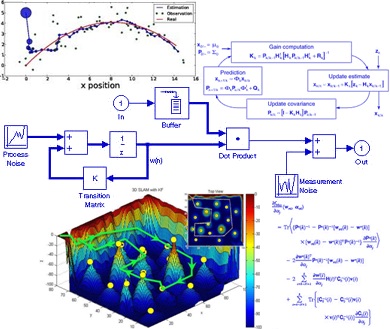 دانلود پاورپوینت فیلتر کالمن بر روی یک سنسور شتاب سنج برای تخمین سه متغیر حالت
دانلود پاورپوینت فیلتر کالمن بر روی یک سنسور شتاب سنج برای تخمین سه متغیر حالت مدار داخلی واکی تاکی(اموزش ساخت)
مدار داخلی واکی تاکی(اموزش ساخت) کتاب افزایش ممبر کانال تلگرام
کتاب افزایش ممبر کانال تلگرام